Biochar is a heterogenous carbonaceous material (EBC, 2012) that consists of two distinct carbon pools with different degrees of persistence when applied to soil:
- The persistent aromatic carbon (PAC) pool, which consists of larger clusters of aromatic carbon rings (see figure 1), generally with more than seven aromatic rings, is not susceptible to degradation. The PAC pool has a mean residence time (MRT) in soil largely exceeding 1000 years (Bowring et al., 2022; Howell et al., 2022), independent of common environmental factors such as soil humidity, temperature, freeze-thaw-cycles, and biological activity or agricultural practices like tillage. Cluster sizes of aromatic carbons (how the carbon ring structures cling together) may be more important for persistence than the sheer number of aromatic rings in a molecule (Mao et al., 2012; McBeath & Smernik, 2009; Nguyen et al., 2010).
- The semi-persistent carbon (SPC) pool, which contains aliphatic, small aromatic, and heteroaromatic carbon species, is more degradable in soil (Rombolà et al., 2016). Some compounds of the semi-persistent carbon pool can degrade within the first year after soil application; others will persist for decades and even centuries depending on the chemistry of the aliphatic and small aromatic compounds and their physical placement within the porous structure of the biochar. On average, the SPC fraction of biochar has an MRT in the order of at least 50 to 100 years, depending on the biochar composition (i.e., distribution of aliphatic carbon, small aromatics, heteroaromatics), the soil type, and the climate (Bowring et al., 2022; Hilscher & Knicker, 2011; Lehmann et al., 2015; Pisani et al., 2014; Schmidt et al., 2011; Singh et al., 2012). With an MRT of 49 years for soil organic carbon (Schmidt et al., 2011), considering the evidence that pyrogenic carbon persists longer in soil than soil organic carbon (S. Lutfalla et al., 2017; Schmidt et al., 2011) and the calculated MRT of 91 years for 49% of soil-applied pyrogenic carbon in six field trials with none optimized biochars, the application of an MRT of 50 years for the semi-persistent carbon pool of biochar with an H:C < 0.4 is conservative and presents a considerable margin.
In the environment, each of the carbonaceous compounds separated in those two respective carbon pools shows distinct degradation dynamics that can be described by an individual degradation curve. If biochar is incubated for one or two, or even eight years, as done in controlled incubation laboratory studies (Kuzyakov et al., 2014; Lehmann et al., 2015) and the resulting degradation data are then mathematically extrapolated into the far future, the prediction of the degradation dynamic is erroneous because it assumes that (1) the biochar consists only of semi-persistent carbon pools (aliphatics and small clusters of aromatic and heteroaromatic rings) and (2) the exponential biological degradation model is valid, despite degradation mechanisms being rather physico-chemical than biological. Over a time scale of several thousands of years, persistent aromatic carbon moieties may eventually also be degraded (Bowring et al., 2022), but this fact is barely contained in degradation data measured only during the first decade after biochar was incubated in soil.
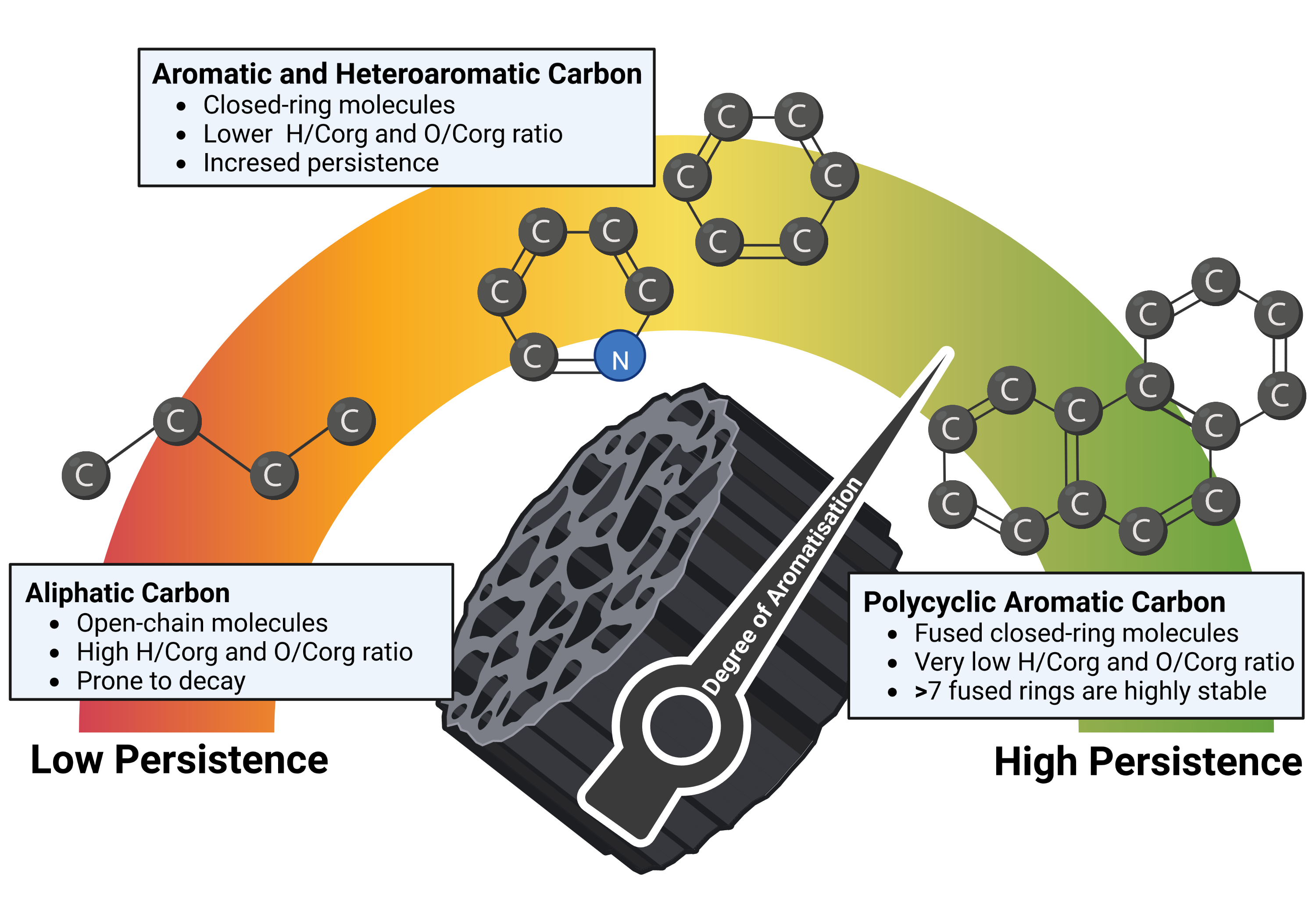
Figure 1: Schematic representation of different molecular forms of carbon in biochar.
All biochar incubation studies observed that the rate of degradation slows down exponentially with time and that the experimental data can be fitted mathematically with bi- or trimodal decay functions (Lehmann et al., 2015; Wang et al., 2016; Zimmerman & Gao, 2013). However, such mathematical fitting may be misleading as it cannot account for qualitative transitions (e.g., removal of physical protection of compounds inside the biochar structure) occurring decades and centuries after the latest measured data points. As shown by Lutfalla et al. (2019), the small number of existing data sets presenting decadal degradation data of carbon in soil cannot be fitted by such bi- or trimodal decay functions. Therefore, projecting the degradation behavior of the semi-persistent carbon pool onto the degradation curve of the entire biochar is not adequate and biases our understanding of long-term carbon dynamics.
The percentage of PAC in a given biochar depends mainly on the pyrolysis conditions (i.e., temperature, residence time, heating rate, particle size, carrier gas, pressure) but also on the feedstock characteristics (i.e., lignin and ash content of biomass) (McDonald-Wharry, 2021). The PAC content can be quantified by hydrogen pyrolysis (HyPy) (Ascough et al., 2009; Rombolà et al., 2016) or by Raman spectroscopy (McDonald-Wharry, 2021; McDonald-Wharry et al., 2013). HyPy analysis is reliable and proven but too complex to be utilized in commercial labs and thus too expensive to be used in routine analysis as for example, in the EBC certification process. Raman spectroscopy as well as the newer Mid-Infrared or Rock-Eval methods, are more cost-efficient analytical methods and are currently under methodological evaluation for the EBC.
Other parameters of biochar, such as production conditions (e.g., temperature) and elemental composition (i.e., using molar H:C and O:C ratio), which are frequently used as proxies for the degree of aromatization rely on poorly constrained databases with low analytical quality. The dataset used e.g., by Woolf et al. (2021) and the IPCC (IPCC, 2019) to calculate biochar persistence based on the highest treatment temperature (HTT) and H:C proxies, contained only one biochar series where HTT was actually measured (Budai et al., 2014) all other temperatures were estimates. Pyrolysis temperature, i.e., the actual temperature inside the biomass particle during conversion, cannot be measured accurately in most industrial pyrolyzers and does not capture the effects that heating rate, residence time, particle size, and pressure have on the formation of PAC (Santín et al., 2017). Moreover, when using literature data on H:C to parameterize mathematical functions on biochar persistence, more care needs to be taken to only use correctly analyzed carbon and hydrogen contents of materials that are really biochar. The IPCC (2019) and Woolf (2021) included e.g. many materials with H:C ratios above 0.7 which are clearly not biochars (EBC, 2012; IBI, 2015) and considered implausible data resulting from insufficiently described analyses (e.g., how was the biochar dried before measuring the H-content). While the molar H:C ratio can be measured with sufficient precision, some fractions of biochars with low H:C are not necessarily PAC and may be considered semi-persistent only (Howell et al., 2022). The H:C ratio is thus a proxy with limited significance for the quantification of biochar persistence.
Since PAC is not yet analyzed for every EBC-certified biochar, average biochar data from the literature should be used with caution. Conservative security margins must be used to estimate the persistent biochar content and, thus, the portion of biochar carbon that will endure as a C-sink for more than 1000 years. Based on the degradation experiments published so far and considering that the calculated decay functions express only the degradation dynamic of the semi-persistent biochar carbon pool, the carbon calculated as remaining after 100 years is regarded as the minimum PAC fraction of biochars with an H:C ratio < 0.4. Applying the conventionally assumed average degradation rate of 0.3% per year, the 74% of carbon remaining after 100 years (Camps-Arbestain et al., 2015; H.-P. Schmidt et al., 2020) can be considered PAC. This corresponds well to the experimental data presented by Howell et al. (2022), finding 75% stable polycyclic aromatic carbon for various engineered biochars with H:C ratios below 0.4 using the HyPy quantification method. Biochars with H:C ratios above 0.4 are likely to have a distinct labile carbon pool of incomplete pyrolyzed biomass or condensates that may become subject to more rapid biological degradation. Dedicated research for those materials is needed to assess the persistence of pyrolyzed biomass presenting high H:C ratios above 0.4 (Pulcher et al., 2022).
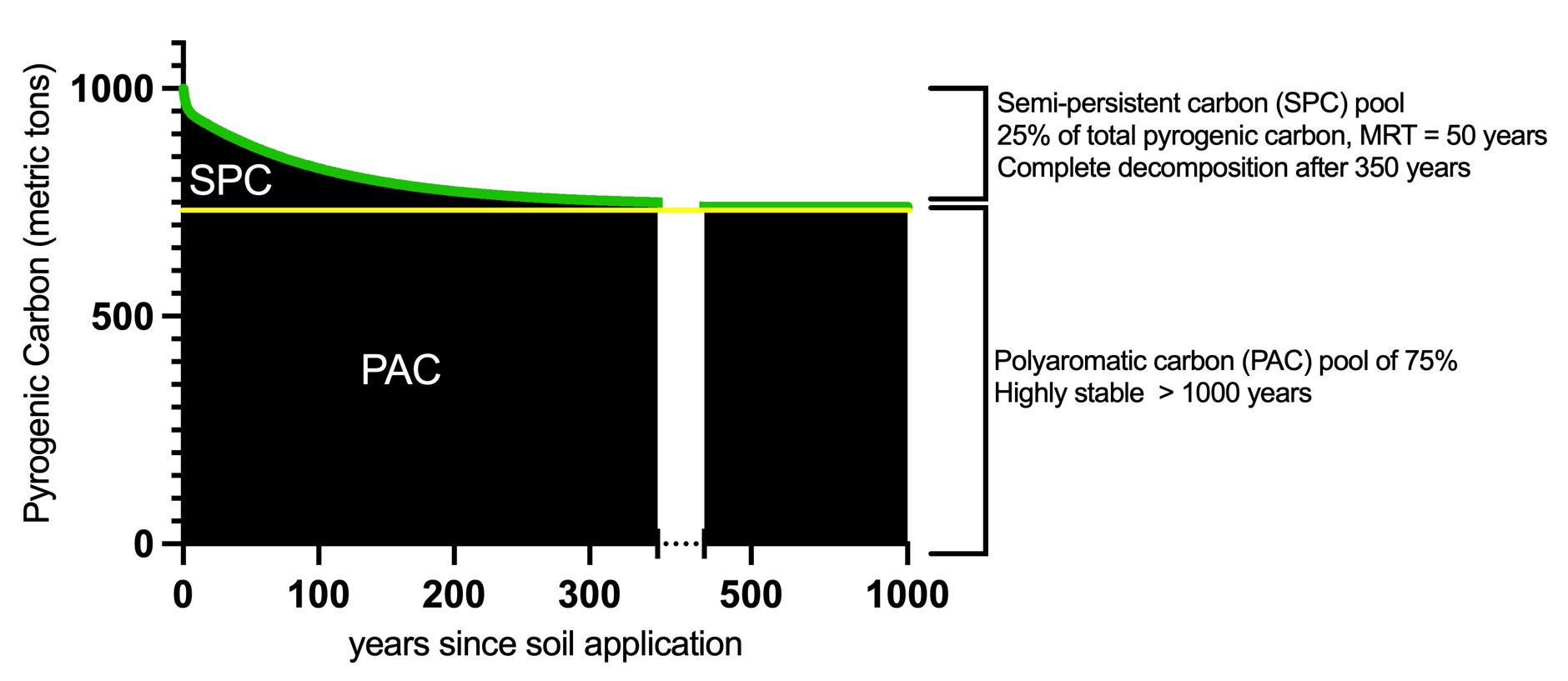
Fig. 2: Sequestration curve of a 1000 tons carbon sink made from soil-applied biochar with an H:C ratio below 0.4. The persistent aromatic carbon (PAC) pool presents 750 t carbon that will remain over more than 1000 years in the terrestrial system. The semi-persistent carbon (SPC) pool has a minimum MRT of 50 years and was modeled on a bi-modal exponential decay function. The complete SPC decay occurs over 350 years. Thus, the total carbon sink decreases to 87.5% after 50 years and reaches the stable PAC plateau of 75.0% of total pyrogenic carbon after 350 years. The decay function is
〖Total PyC〗_((x))=a*e^((-kf*x) )+b*e^((-ks*x) )+P
with a = 45.423, kf = 0.513, b = 212.007, ks = 0.009448, P = 742.5 nd x = year after soil application. The decay curve of the semi-persistent carbon pool is an approximation covering multiple discrete (physical) degradation events rather than a continually harmonious decomposition.
To the best of the current scientific knowledge, it is safe to assume that biochar with an H:C ratio below 0.4 can be best described by a 2-pool-model presenting
- A persistent aromatic carbon (PAC) pool of 75% with an MRT of >1000 (1400-14400 years, see annex), competitive with geological carbon storage and suitable for CO2-emission compensation and
- A semi-persistent carbon (SPC) pool with an MRT of at least 50 years offering an additional, valuable climate cooling service, yet of a different quality than persistent aromatic carbon (PAC).
Annex 1
Mean residence times of natural pyrogenic carbon
Calculations of global inputs and deposits of naturally produced pyrogenic carbons (PyC) can attest to how robust and conservative the assumption of these average persistence rates over 100 years is. Forest, bush, and steppe fires are examples of incomplete combustion, which transform part of the biomass into chars, i.e., PyC. According to recent surveys of natural fires, 5-15% of the biomass carbon involved in the fire is converted to PyC (Santín et al., 2016). Natural PyCs are similar in structure and material properties to industrially produced biochar. However, it can be assumed that the stability of high HTT industrial biochar, and thus the mean residence time (MRT), is even higher than that of natural PyC (Howell et al., 2022; Santín et al., 2017) due to more controlled and homogeneous high-temperature conditions.
Mainly through forest and steppe fires, about 0.114-0.383 Gt (Giga tons) of pyrogenic carbon (PyC) are generated each year (Santín et al., 2016). Globally, the total mass of PyC in soils is 71-212 Gt, in nearshore sediments 400-1200 Gt, and in further ocean sediments 80-240 Gt (Bird et al., 2015; Santín et al., 2016), resulting in a global PyC pool of 550-1,650 Gt (excluding PyC in water bodies and groundwater sediments). Based on the dimension of the global PyC pool and the annual input of PyC of 0.114 - 0.383 Gt given above, the average MRT of natural PyC can be calculated as
MRT = (GlobalPyC-pool)/annualPyCinput
The MRT range of natural PyC could thus be calculated as (550 Gt / 0.383 Gt a-1 to 1,650 Gt / 0.114 Gt a-1 =) 1,440 to 14,500 years. This time frame is confirmed by Bowring et al. (2022), who determined a minimum MRT of 2,760 years using the same data basis but without including sedimentary PyC.
If we use the extrapolation of Reisser et al. (2016), according to which the PyC content of soil organic carbon (SOC) is 14%, and the global content of SOC is 1,500 to 3,000 Gt (Scharlemann et al., 2014) the global PyC content in soils would be about 210 - 420 Gt (Leifeld et al., 2018). From the annual PyC input of 0.114 - 0.383 Gt, the MRT for PyC in soils would be (210 Gt / 0.382 Gt a-1 to 420 Gt / 0.114 Gt a-1) 550 to 3,700 years. Since the MRT of PyC in sediments is significantly higher than in soils, the difference between the two calculations is plausible. Note, however, that most of the PyC in nearshore sediments is originally derived from PyC leached from soils (Coppola & Druffel, 2016), so that much longer MRTs than the calculated 550 to 3,700 years would result for soil-PyC, except that the pyrogenic carbon would no longer be found in soils but as deposits in sediments (Coppola et al., 2014).
References
Abiven, S., Hengartner, P., Schneider, M. P. W., Singh, N., & Schmidt, M. W. I. (2011). Pyrogenic carbon soluble fraction is larger and more aromatic in aged charcoal than in fresh charcoal. Soil Biology and Biochemistry, 43(7), 1615–1617. https://doi.org/10.1016/j.soilbio.2011.03.027
Ascough, P. L., Bird, M. I., Brock, F., Higham, T. F. G., Meredith, W., Snape, C. E., & Vane, C. H. (2009). Hydropyrolysis as a new tool for radiocarbon pre-treatment and the quantification of black carbon. Quaternary Geochronology, 4(2), 140–147. https://doi.org/10.1016/j.quageo.2008.11.001
Bird, M. I., Wynn, J. G., Saiz, G., Wurster, C. M., & McBeath, A. (2015). The Pyrogenic Carbon Cycle. Annual Review of Earth and Planetary Sciences, 43(1), 273–298. https://doi.org/10.1146/annurev-earth-060614-105038
Bowring, S. P. K., Jones, M. W., Ciais, P., Guenet, B., & Abiven, S. (2022). Pyrogenic carbon decomposition critical to resolving fire's role in the Earth system. Nature Geoscience 2022 15:2, 15(2), 135–142. https://doi.org/10.1038/S41561-021-00892-0
Budai, A., Wang, L., Gronli, M. G., Strand, L. T., Antal, M. J., Abiven, S., Dieguez-Alonso, A., Anca-Couce, A., & Rasse, D. P. (2014). Surface Properties and Chemical Composition of Corncob and Miscanthus Biochars: Effects of Production Temperature and Method. Journal of Agricultural and Food Chemistry, 62(17), 3791–3799. https://doi.org/10.1021/jf501139f
Camps-Arbestain, M., Amonette, J. E., Singh, B., Wang, T., & Schmidt, H.-P. (2015). A biochar classification system and associated test methods. In J. Lehmann & S. Joseph (Eds.), Biochar for environmental management (earthscan, pp. 165–194). Routledge.
Coppola, A. I., & Druffel, E. R. M. (2016). Cycling of black carbon in the ocean. Geophysical Research Letters, 43(9), 4477–4482. https://doi.org/10.1002/2016GL068574
Coppola, A. I., Ziolkowski, L. A., Masiello, C. A., & Druffel, E. R. M. (2014). Aged black carbon in marine sediments and sinking particles. Geophysical Research Letters, 41(7), 2427–2433. https://doi.org/10.1002/2013GL059068
EBC. (2012). European Biochar Certificate - Guidelines for a Sustainable Production of Biochar. Version 7.1 of 22th December 2015. European Biochar Foundation; European Biochar Foundation (EBC). https://doi.org/10.13140/RG.2.1.4658.7043
Hilscher, A., & Knicker, H. (2011). Degradation of grass-derived pyrogenic organic material, transport of the residues within a soil column and distribution in soil organic matter fractions during a 28month microcosm experiment. Organic Geochemistry, 42(1), 42–54. https://doi.org/10.1016/j.orggeochem.2010.10.005
Howell, A., Helmkamp, S., & Belmont, E. (2022). Stable polycyclic aromatic carbon (SPAC) formation in wildfire chars and engineered biochars. Science of The Total Environment, 849, 157610. https://doi.org/10.1016/J.SCITOTENV.2022.157610
IBI. (2015). Standardized product definition and product testing guidelines for biochar that is used in soil, v. 1.1. International Biochar Initiative, January 2011, 1–47. https://doi.org/http://www.biochar-international.org/characterizationstandard. 22
IPCC. (2019). Method for estimating the change in mineral soil organic carbon stocks from biochar amendments: basis for future methodological development. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories (p. Ap4.1). IPCC. https://www.ipcc-nggip.iges.or.jp/public/2019rf/pdf/4_Volume4/19R_V4_Ch02_Ap4_Biochar.pdf
Kuzyakov, Y., Bogomolova, I., & Glaser, B. (2014). Biochar stability in soil: Decomposition during eight years and transformation as assessed by compound-specific 14C analysis. Soil Biology and Biochemistry, 70, 229–236. https://doi.org/10.1016/j.soilbio.2013.12.021
Lehmann, J., Abiven, S., Kleber, M., Pan, G., Singh, B. P., Sohi, S. P., & Zimmerman, A. R. (2015). Persistence of biochar in soil. In J. Lehmann & S. D. Joseph (Eds.), Biochar for environmental management (Routledge, pp. 235–282).
Leifeld, J., Alewell, C., Bader, C., Krüger, J. P., Mueller, C. W., Sommer, M., Steffens, M., & Szidat, S. (2018). Pyrogenic Carbon Contributes Substantially to Carbon Storage in Intact and Degraded Northern Peatlands. Land Degradation & Development, 29(7), 2082–2091. https://doi.org/10.1002/ldr.2812
Lutfalla, S., Abiven, S., Barré, P., Wiedemeier, D. B., Christensen, B. T., Houot, S., Kätterer, T., Macdonald, A. J., Van Oort, F., & Chenu, C. (2017). Pyrogenic carbon lacks long-term persistence in temperate arable soils. Frontiers in Earth Science. https://doi.org/10.3389/feart.2017.00096
Lutfalla, Suzanne, Barré, P., Bernard, S., Le Guillou, C., Alléon, J., & Chenu, C. (2019). Multidecadal persistence of organic matter in soils: Multiscale investigations down to the submicron scale. Biogeosciences, 16(7), 1401–1410. https://doi.org/10.5194/BG-16-1401-2019
Mao, J.-D., Johnson, R. L., Lehmann, J., Olk, D. C., Neves, E. G., Thompson, M. L., & Schmidt-Rohr, K. (2012). Abundant and Stable Char Residues in Soils: Implications for Soil Fertility and Carbon Sequestration. Environmental Science & Technology, 46(17), 9571–9576. https://doi.org/10.1021/es301107c
McBeath, A. V., & Smernik, R. J. (2009). Variation in the degree of aromatic condensation of chars. Organic Geochemistry, 40(12), 1161–1168. https://doi.org/10.1016/j.orggeochem.2009.09.006
McDonald-Wharry, J. (2021). 2013–2014 Survey of Chars Using Raman Spectroscopy. C 2021, Vol. 7, Page 63, 7(3), 63. https://doi.org/10.3390/C7030063
McDonald-Wharry, J., Manley-Harris, M., & Pickering, K. (2013). Carbonisation of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy. Carbon, 59, 383–405. https://doi.org/10.1016/J.CARBON.2013.03.033
Nguyen, B. T., Lehmann, J., Hockaday, W. C., Joseph, S., & Masiello, C. A. (2010). Temperature Sensitivity of Black Carbon Decomposition and Oxidation. Environmental Science & Technology, 44(9), 3324–3331. https://doi.org/10.1021/es903016y
Pisani, O., Hills, K. M., Courtier-Murias, D., Haddix, M. L., Paul, E. A., Conant, R. T., Simpson, A. J., Arhonditsis, G. B., & Simpson, M. J. (2014). Accumulation of aliphatic compounds in soil with increasing mean annual temperature. Organic Geochemistry, 76, 118–127. https://doi.org/10.1016/J.ORGGEOCHEM.2014.07.009
Pulcher, R., Balugani, E., Ventura, M., Greggio, N., & Marazza, D. (2022). Inclusion of biochar in a C dynamics model based on observations from an 8-year field experiment. SOIL, 8(1), 199–211. https://doi.org/10.5194/SOIL-8-199-2022
Rombolà, A. G., Fabbri, D., Meredith, W., Snape, C. E., & Dieguez-Alonso, A. (2016). Molecular characterization of the thermally labile fraction of biochar by hydropyrolysis and pyrolysis-GC/MS. Journal of Analytical and Applied Pyrolysis, 121, 230–239. https://doi.org/10.1016/J.JAAP.2016.08.003
Santín, C., Doerr, S. H., Kane, E. S., Masiello, C. A., Ohlson, M., de la Rosa, J. M., Preston, C. M., & Dittmar, T. (2016). Towards a global assessment of pyrogenic carbon from vegetation fires. Global Change Biology, 22(1), 76–91. https://doi.org/10.1111/gcb.12985
Santín, C., Doerr, S. H., Merino, A., Bucheli, T. D., Bryant, R., Ascough, P., Gao, X., & Masiello, C. A. (2017). Carbon sequestration potential and physicochemical properties differ between wildfire charcoals and slow-pyrolysis biochars. Scientific Reports, 7(1), 11233. https://doi.org/10.1038/s41598-017-10455-2
Scharlemann, J. P., Tanner, E. V., Hiederer, R., & Kapos, V. (2014). Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Management, 5(1), 81–91. https://doi.org/10.4155/cmt.13.77
Schmidt, H.-P., Hagemann, N., & Kammann, C. I. (2020). Guidelines for the certification of the carbon sink potential of biochar (Version 1.0). https://www.european-biochar.org/media/doc/26
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., Kleber, M., Kögel-Knabner, I., Lehmann, J., Manning, D. A. C., Nannipieri, P., Rasse, D. P., Weiner, S., & Trumbore, S. E. (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478(7367), 49–56. https://doi.org/10.1038/nature10386
Singh, N., Abiven, S., Torn, M. S., & Schmidt, M. W. I. (2012). Fire-derived organic carbon in soil turns over on a centennial scale. Biogeosciences, 9(8), 2847–2857. https://doi.org/10.5194/bg-9-2847-2012
Wang, J., Xiong, Z., & Kuzyakov, Y. (2016). Biochar stability in soil: meta‐analysis of decomposition and priming effects. GCB Bioenergy, 8(3), 512–523. https://doi.org/10.1111/gcbb.12266
Zimmerman, A. R., & Gao, B. (2013). The Stability of Biochar in the Environment. In N. Ladygina & F. Rineau (Eds.), Biochar and Soil Biota (crc press, pp. 1–40).

- Please write us your comment -
×