The Carbon Cycle(s)
The carbon cycle can be divided into a fast and a slow cycle. The fast cycle encompasses the atmosphere, the biosphere (all plants and living beings), the pedosphere (the upper two meters of soil), the hydrosphere (oceans, lakes, rivers), and the anthroposphere (the built environment). Carbon moves continuously between those different spheres, either as CO2, bicarbonate, or in the form of organic carbon. The average circulation or mean residence time is between several years to several decades. The slow carbon cycle includes, in addition to the carbon spheres of the fast cycle, the lithosphere (rocks, deep sediments, fossils). The lithosphere is home to the so-called geological carbon sinks, which consist mainly of carbonate rock and fossil oil, gas, and coal. The carbon circulation between the lithosphere and the other carbon spheres is in the order of several thousand to hundreds of million years. It includes, e.g., the formation of carbonate sediments and CO2 emissions of volcanos. The formation of organic carbon deposits that formed, e.g., coal, was widely limited to the Carboniferous, a geological period about 300 million years ago, and does not play a considerable role in today's carbon cycle.
Due to human activities, about 500 Gt carbon, which equals 1800 Gt CO2 (1800 billion tons CO2), has been transferred from the slow carbon cycle (i.e., from geological carbon sinks formed in the Carboniferous) into the fast carbon cycle.
The fast carbon cycle is a phenomenon that can be easily understood with everyday observations: Plants consist of around 50% carbon. When they grow in the garden, they extract the carbon that they require from the atmosphere as CO2. When we then eat the garden vegetables, we digest and metabolize the carbon compounds. Some of the digested carbon is incorporated into the cells, and some is excreted. However, most of it is exhaled as CO2. The average person exhales around 1 kg of CO2 per day. The parts of plants not used for food end up on the compost heap, where they are broken down and digested by various organisms, and here, too, CO2 is released into the atmosphere. A portion of the carbon remains as compost, which is worked back into the garden bed. Just like the roots that remain in the soil, this carbon stays in the soil for a bit longer, but after a few years, these molecules are also broken down by microorganisms, and the carbon returns to the atmosphere as CO2. A small proportion of the carbon bound in the soil gets washed out with the groundwater and surface water and ends up in the sea, from where it is only released back into the atmosphere much later.
With this picture in mind, it quickly becomes apparent that when we burn fossil carbon, it does not simply go into the atmosphere but is distributed from there within decades into the various temporary sinks of the fast carbon cycle: the atmosphere, the biosphere, the pedosphere, and the hydrosphere.
In the last 250 years, only a tiny proportion of the fast-cycling carbon entered the slow carbon cycle via carbonization through forest and steppe fires, biochar making, silicate rock weathering, and other geodynamic processes that make the carbon again part of the geology from which the fossil raw materials were extracted. The imbalance of the carbon cycle comes from the massive transfer of carbon from geological storage into the fast carbon cycle, where it increases the CO2 content of the atmosphere and causes global warming.
Fossil carbon in the atmosphere
In 1750, at the beginning of industrialization, the CO2 content of the atmosphere was 280 ppm. Since then, human activities have emitted around 1,800 Gt of CO2 from burning fossil fuels and cement production (IPCC, 2022; Knutti & Rogelj, 2015). At the end of 2023, the CO2 concentration reached 420 ppm. Due to anthropogenic emissions, the CO2 content of the atmosphere has therefore increased by (100% - 280 ppm / 420 ppm =) 33 %.
The atmosphere has a mass of 5.15 * 1015 t (5.15 quadrillion tons = 5.15 Pt). The additional carbon in the atmosphere corresponds to:
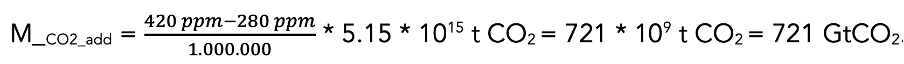
Carbon dioxide does not decay in the atmosphere but can only be removed by being transferred into other carbon pools (biosphere, soil, and oceans). Of the 1,800 Gt of fossil CO2 emitted, at least 1080 GtCO2e have therefore already been transferred to other natural carbon reservoirs.
Although very high biogenic CO2 emissions of (760 ± 220 GtCO2) have also been caused by deforestation, drainage of wetlands, overbuilding, and similar land use changes (IPCC, 2022), the C content of the biosphere and the humus content of soils have remained roughly the same on a global average over the last 250 years (Reichle, 2023). This can be explained by higher biomass productivity in the agricultural sector and the fertilizing effect of higher CO2 levels and nitrogen deposition in natural systems. The amount of biogenic carbon incorporated into the anthroposphere (e.g., the use of wood in the built environment) has increased significantly in recent centuries (Elhacham et al., 2020; Sandak, 2023) but is still negligible compared to global carbon flows. It can, therefore, be assumed that of the approximately 1800 GtCO2 that has increased the carbon content in the short carbon cycle (atmosphere, biosphere, anthroposphere, soil, and oceans) due to fossil emissions, 40% (720 GtCO2) increased atmospheric levels and 60% (1080 GtCO2e) increased levels in the hydrospheric CO2 concentration. However, as we will see in the following chapter, the total increase of CO2e in the ocean and the atmosphere does not mean that all of its additional carbon is of fossil origin because the carbon is intensely mixed between the different spheres of the fast carbon cycle.
The distribution of fossil CO2 within the short carbon cycle
During the last centuries, the oceans have absorbed an additional 1080 Gt CO2e and now store a total of around 3000 Gt CO2e (Gruber et al., 2019), which corresponds to roughly 60% of the anthropogenic fossil emissions. The biosphere contains around 2000 Gt CO2e in the form of organic carbon in plants, algae, animals, and microorganisms (c.f., Figure 1; Bar-On et al., 2018). The soil (0 – 2 m below the earth's surface), also known as the pedosphere, contains around 8800 Gt CO2e in the form of humus, dead plant parts, bones, etc. (Batjes, 1996; Beillouin et al., 2023). The biosphere is in direct exchange with the atmosphere through photosynthesis and plants' associated removal of CO2 from the air. Consequently, plants also absorb carbon from the atmosphere, which was initially extracted and burned as oil, coal, or gas from geological deposits. Chemically, fossil and non-fossil CO2 are identical, and its origin makes no difference to the plant. Even scientifically, it is hardly possible to determine whether a single carbon atom that had passed several tours in the small carbon cycle was of fossil origin or not (Keeling, 1979). Larger, coherent units of fossil carbon (e.g., petrol, plastic in waste, or in the exhaust gas of a coal-fired power plant) can be distinguished from biogenic carbon (e.g., wood or straw) by isotope determination (Mohn et al., 2008), but not if the CO2 molecules were in a previous cycle part of the atmospheric gas mixture.
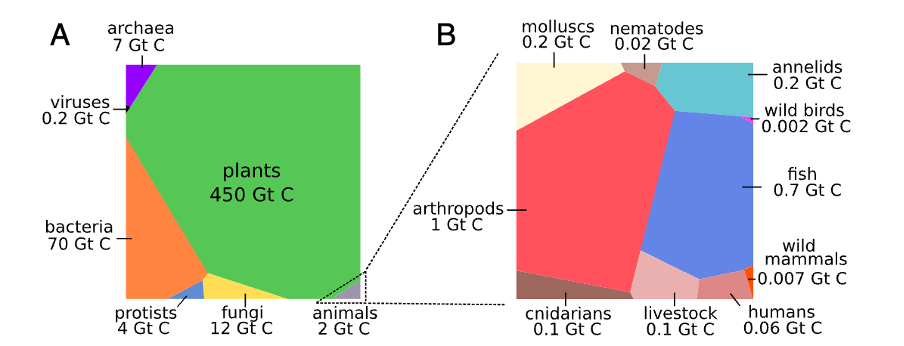
Figure 1: Graphical representation of the global distribution of carbon in biomass in major taxonomic groups; A, where the area of each cell is proportional to the global biomass of the respective taxa; B, carbon in the biomass of different animal taxa, where the contribution of reptiles and amphibians to the total animal biomass is negligible (Bar-On et al., 2018).
Worldwide, plants and algae absorb between 380 and 475 Gt CO2e from the atmosphere every year (Madani et al., 2020). This total biospheric carbon removal is referred to as Gross Primary Productivity (GPP). A significant proportion of this carbon is almost immediately released back into the atmosphere through plant respiration. The actual growth of biomass that preserves the adsorbed carbon for one or several growing seasons is referred to as Net Primary Productivity (NPP) and accumulates around 205 Gt CO2e per year (Ito, 2011; Reichle, 2023). As the total carbon stored in biomass worldwide is around 2000 Gt CO2e, the annual NPP of 205 Gt CO2e results in an average carbon cycle time in the biosphere of (2000 Gt CO2e / 205 GtCO2e * a-1 =) 9.8 years. The biosphere's portion of 2000 Gt CO2e is therefore renewed every 10 years on average. It should be noted that this period is the average over all types of plants, from the short-lived flower on the alpine meadow to the 1000-year-old oak tree that contains carbon from centuries ago. During this average of ten years, all the CO2 in the atmosphere (2160 Gt CO2e) is absorbed and released almost completely (Green & Byrne, 2004). The fast C cycle between the atmosphere and the biosphere functions as a CO2 mixing machine, so to speak.
Due to the approximately 10-year C circulation between the biosphere and the ocean, it can be assumed that the concentration of original fossil carbon in the atmosphere and in the biosphere has become almost the same over the last 50 years. In older trees, some of whose carbon was already absorbed from the atmosphere many years ago when the fossil CO2 concentration in the environment was lower, the average concentration is somewhat lower, but in annual plants, it already corresponds to this year's, i.e. historically highest, fossil CO2 concentration in the atmosphere.
Fossil carbon in the biosphere
With the atmosphere (2200 Gt), biosphere (2000 Gt), pedosphere (8800 Gt), and the oceans (3000 Gt), we are surrounded by around 16,000 Gt of CO2e. Of this, 1800 Gt CO2e are of fossil origin, which represents more than 10% of the total carbon in the fast carbon cycle. For biomass, we have shown that mixing takes around 10 years. In soil, carbon entering from the biosphere mixes more slowly with existing soil organic carbon (SOC). Since the mean residence time of SOC is roughly 50 years (Schmidt et al., 2011), the complete recycling of carbon between the atmosphere, biosphere, and pedosphere can be assumed to take about 60 years (10 years to mix atmospheric C with biosphere C and 50 years for biosphere C with pedosphere C). However, major differences exist between, e.g., tropical rainforests and a subarctic wetland. Significantly faster turnover rates can be measured in warmer and humid climates than in cold and dry ones.
With the rapid carbon exchange between the atmosphere, biosphere, and pedosphere and the previously mentioned fact that the oceans have absorbed nearly 60% of the CO2 that has entered the fast C cycle during the last 250 years, it can be safely assumed that carbon from fossil origin has long since been mixed within all carbon pools of the fast C cycle and that globally, an average of 10% of the fast cycle's carbon is of fossil origin.
Since the exchange between the atmosphere and the biosphere is faster than with the other pools, and the concentration in the atmosphere is highest due to the unabated emissions from fossil fuels, the original concentration of fossil CO2 in the biosphere is significantly higher than 10%. How much higher than 10% the concentration actually is can only be determined imprecisely, as the other pools' exact circulation and mixing times in these enormous dimensions with such large local differences are difficult to determine.
As animals and humans feed mainly from the fastest-growing biomass pool (annual plants and fruits), it can be extrapolated that the fossil C concentration in animal and human cells is roughly equal to the concentration of fossil CO2 in the atmosphere. The fossil C content in animals and humans, in their blood and respiration, is thus also well over 10%. However, to illustrate what it means for our understanding and the C sink economy, we can start from the very conservatively determined minimum value of 10% fossil carbon content in all flora and fauna cells.
As the average human body consists of roughly 12 kg of carbon and at least 10% is of fossil origin, each person contains approximately 1.2 kg of carbon, which was initially stored deep underground as coal, crude oil, or natural gas, burned for our comfort, emitted into the atmosphere, then reabsorbed by plants and finally found its way into our cells via the food chain. We exhale approx. 1 kg of CO2 per day, with the C of the CO2 coming from metabolized food, i.e., originally from plants, which in turn had captured the C from the atmosphere. Over the course of 80 years, a person will expire around (365 d * 1 kg CO2/d * 80 a * 10% =) 3 tons of fossil CO2 back to the atmosphere. Another 4.5 kg CO2 of fossil origin is added as a greenhouse gas during cremation.
Shrub prunings, straw, vegetables, and even liquid manure and sewage sludge have, on average, at least a tenth of their carbon from fossil sources. If this biomass is pyrolyzed, one-tenth of the biochar-based carbon sink is basically of fossil origin, and one-tenth of the pyrolysis gases that are burned and emitted back into the atmosphere as greenhouse gases are also of fossil origin. Now, what does that mean for trading persistent biochar carbon sinks? Should we reduce 10% of its value?
Natural Carbon Purity and the regulation of fossil emissions
In order to gradually reduce emissions from specific industrial sectors, the EU introduced an emissions trading system (EU ETS) in 2005, which was designed according to the "cap and trade" principle: Power plants, coking plants, refineries, and other industrial companies must prove at the end of the year that they purchased emissions certificate (pollution allowance) for every ton of CO2e. These are allocated to the individual companies according to a complex distribution matrix. If they emit less, they can sell surplus certificates to companies that have failed to reduce their emissions sufficiently. While the system creates incentives to reduce emissions, the artificial market trading pollution rights may seem incongruous.
From 2028, waste incineration plants in the EU will also be included in the EU emissions trading scheme. Given that their emissions contain a blend of fossil carbon (e.g., from plastic, polystyrene, nylon, etc.) and biological carbon (e.g., food scraps, wood, paper), complicated and expensive waste gas analyses must be carried out to calculate what proportion of the incinerated waste was derived from fossil raw materials versus biomass. These tests are done on the basis of the isotopic signature of the CO2 in the waste gas. Cement plants that burn secondary fuels such as waste wood or other residual materials have already had to analyze and differentiate the proportions of biogenic and fossil carbon in the same or similar ways for several years. EU emission certificates must be presented for the fossil share; while the share from biomass, instead, is considered climate-neutral and thus free of charge. Although at least 10% of biomass carbon is of fossil origin, as shown above, the reality is that this cannot be determined using the isotope method. And the state cannot tax or regulate what it does not know (or understand).
Shifting the focus: from Exhaust to Extraction
The real question is, however, whether it makes any sense at all to differentiate between fossil and non-fossil CO2 emissions. Doesn't every form of emitted CO2 contribute to climate change? Why should biomass, paper, paper cups, food, and leather bags be burned or left to rot free of tax or duty, while plastic bags, polystyrene, and car tires are subject to charges? Why are we fixing the matter at the chimney exhaust and not at the place where carbon is extracted from the earth's carbon vault (the geological C-sink)?
All biomass and, therefore, all biochar contain more than 10% carbon of fossil origin, yet no one is demanding that this be deducted from the carbon sinks. And no one has ever demanded that DACCS companies like Climeworks refund part of the carbon credits because more than 10% of the CO2 removed from the atmosphere was of fossil origin. This difference in tracking and tolling fossil carbon is only now being made when it comes to waste management.
Wouldn't it be better to apply the tax or ban at the point where fossil carbon is extracted from geological deposits? The geological carbon sink is destroyed the moment oil, natural gas, and coal are extracted from geologically safe deposits and fed into the fast carbon cycle. The true damage is done when this additional (fossil) carbon enters the anthroposphere, atmosphere, biosphere, and oceans - be it directly as CO2 from a power plant or as a temporary carbon used in the form of a plastic window frame, a broomstick, or a coffee cup that will pass back into the atmosphere soon thereafter.
In order to rebalance carbon levels and restore climate, the destruction of geological carbon sinks must be stopped, and CO2 emissions, whether from biomass or fossil fuels, must be significantly reduced through carbon recycling directly at exhaust stacks. Once the fossil deposits have been exploited, it no longer makes sense to differentiate between fossil and "biological" carbon. Saving the climate means (1) preventing greenhouse gases from reaching the atmosphere, regardless of their origin, and (2) efficiently removing CO2 from the atmosphere and using and thus (temporarily) storing it in new carbon sink materials (see our previous article of the present tBJ series. Carbon harvested from the short-term carbon cycle will not only replace fossil carbon as one of the chemical industry’s primary raw materials but also offer the potential to replenish the geological carbon storage in the lithosphere and oil fields. Follow our upcoming articles in this tBJ series to discover carbon’s future.
Literature
Bar-On, Y. M., Phillips, R., & Milo, R. (2018). The biomass distribution on Earth. Proceedings of the National Academy of Sciences of the United States of America, 115(25), 6506–6511. https://doi.org/10.1073/PNAS.1711842115/SUPPL_FILE/1711842115.SAPP.PDF
Batjes, N. H. (1996). Total carbon and nitrogen in the soils of the world. European Journal of Soil Science, 47(2), 151–163. https://doi.org/10.1111/J.1365-2389.1996.TB01386.X
Beillouin, D., Corbeels, M., Demenois, J., Berre, D., Boyer, A., Fallot, A., Feder, F., & Cardinael, R. (2023). A global meta-analysis of soil organic carbon in the Anthropocene. Nature Communications 2023 14:1, 14(1), 1–10. https://doi.org/10.1038/s41467-023-39338-z
Elhacham, E., Ben-Uri, L., Grozovski, J., Bar-On, Y. M., & Milo, R. (2020). Global human-made mass exceeds all living biomass. Nature 2020 588:7838, 588(7838), 442–444. https://doi.org/10.1038/s41586-020-3010-5
Green, C., & Byrne, K. A. (2004). Biomass: Impact on Carbon Cycle and Greenhouse Gas Emissions. Encyclopedia of Energy, 223–236. https://doi.org/10.1016/B0-12-176480-X/00418-6
Gruber, N., Clement, D., Carter, B. R., Feely, R. A., van Heuven, S., Hoppema, M., Ishii, M., Key, R. M., Kozyr, A., Lauvset, S. K., Monaco, C. Lo, Mathis, J. T., Murata, A., Olsen, A., Perez, F. F., Sabine, C. L., Tanhua, T., & Wanninkhof, R. (2019). The oceanic sink for anthropogenic CO 2 from 1994 to 2007. Science, 363(6432), 1193–1199. https://doi.org/10.1126/SCIENCE.AAU5153/SUPPL_FILE/AAU5153_GRUBER_SM.PDF
IPCC. (2022). IPCC sixth assessment report (AR6) - Working group III contribution. In UNEP: Vol. III. https://report.ipcc.ch/ar6wg3/pdf/IPCC_AR6_WGIII_FinalDraft_FullReport.pdf
Ito, A. (2011). A historical meta-analysis of global terrestrial net primary productivity: are estimates converging? Global Change Biology, 17(10), 3161–3175. https://doi.org/10.1111/J.1365-2486.2011.02450.X
Keeling, C. D. (1979). The Suess effect: 13Carbon-14Carbon interrelations. Environment International, 2(4–6), 229–300. https://doi.org/10.1016/0160-4120(79)90005-9
Knutti, R., & Rogelj, J. (2015). The legacy of our CO2 emissions: a clash of scientific facts, politics and ethics. Climatic Change, 133(3), 361–373. https://doi.org/10.1007/s10584-015-1340-3
Madani, N., Parazoo, N. C., Kimball, J. S., Ballantyne, A. P., Reichle, R. H., Maneta, M., Saatchi, S., Palmer, P. I., Liu, Z., & Tagesson, T. (2020). Recent Amplified Global Gross Primary Productivity Due to Temperature Increase Is Offset by Reduced Productivity Due to Water Constraints. AGU Advances, 1(4), e2020AV000180. https://doi.org/10.1029/2020AV000180
Mohn, J., Szidat, S., Fellner, J., Rechberger, H., Quartier, R., Buchmann, B., & Emmenegger, L. (2008). Determination of biogenic and fossil CO2 emitted by waste incineration based on 14CO2 and mass balances. Bioresource Technology, 99(14), 6471–6479. https://doi.org/10.1016/J.BIORTECH.2007.11.042
Reichle, D. E. (2023). The Global Carbon Cycle and Climate Change: Scaling Ecological Energetics (Elsevier). Candice Janko.
Sandak, A. (2023). Engineered living materials for sustainable and resilient architecture. Nature Reviews Materials, 8(6), 357–359. https://doi.org/10.1038/S41578-023-00554-0
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., Kleber, M., Kögel-Knabner, I., Lehmann, J., Manning, D. A. C., Nannipieri, P., Rasse, D. P., Weiner, S., & Trumbore, S. E. (2011). Persistence of soil organic matter as an ecosystem property. Nature, 478(7367), 49–56. https://doi.org/10.1038/nature10386

- Please write us your comment -
×